electron configuration of arsenic|Arsenic electron configuration : Clark Learn how to write the full and shorthand electron configuration for a neutral arsenic atom using the Aufbau diagram. See the answer, explanation and alternative expressions for As. How to convert Philippine pesos to US dollars. 1 Input your amount. Simply type in the box how much you want to convert. 2 Choose your currencies. Click on the dropdown to select PHP in the first dropdown as the currency that you want to convert and USD in the second drop down as the currency you want to convert to.
PH0 · What is the electron configuration for As?
PH1 · How to write the electron configuration for Arsenic (As and the As
PH2 · Electron configuration of arsenic 【Electron Configuration】 2022
PH3 · Electron Configuration for Arsenic (As, As3
PH4 · Arsenic electron configuration
PH5 · Arsenic Electron Configuration (As) with Orbital Diagram
PH6 · Arsenic (As) [33] — Chemical Element — Periodic Table
PH7 · Arsenic (As)
PH8 · Arsenic
𝙉𝙤 𝙘𝙤𝙥𝙮𝙧𝙞𝙜𝙝𝙩 𝙞𝙣𝙛𝙧𝙞𝙣𝙜𝙚𝙢𝙚𝙣𝙩 𝙞𝙣𝙩𝙚𝙣𝙙𝙚𝙙 𝙛𝙤𝙧 𝙚𝙣𝙩𝙚𝙧𝙩𝙖𝙞𝙣𝙢𝙚𝙣𝙩 𝙤𝙣𝙡𝙮. Pinay Rare Scandal 77.4K members. 𝙉𝙤 𝙘𝙤𝙥𝙮𝙧𝙞𝙜𝙝𝙩 𝙞𝙣𝙛𝙧𝙞𝙣𝙜𝙚𝙢𝙚𝙣𝙩 𝙞𝙣𝙩𝙚𝙣𝙙𝙚𝙙 𝙛𝙤𝙧 .
electron configuration of arsenic*******The ground state electron configuration of arsenic is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3. This electron configuration shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenicare five. The elements that have 5, 6, or 7 electrons in the last shell receive the . Tingnan ang higit paThe total number of electrons in arsenic is thirty-three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in arsenic in . Tingnan ang higit pa
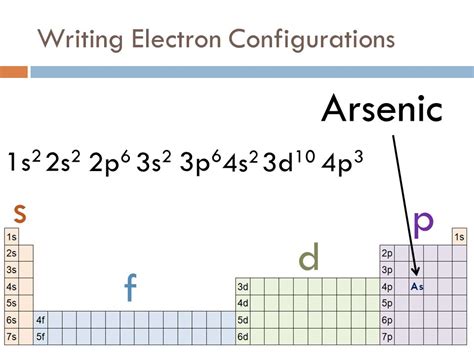
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit pa Learn how to write the full and shorthand electron configuration for a neutral arsenic atom using the Aufbau diagram. See the answer, explanation and alternative expressions for As.Arsenic electron configuration A step-by-step description of how to write the electron configuration for Arsenic (As and the As 3- ion).In order to write the As electron configuration we .electron configuration of arsenic The information on this page is fact-checked. Arsenic electron configuration | Image: Learnool. The arsenic electron configuration, represented as [ Ar] 4s 2 3d 10 4p . What is The Electron Configuration of Arsenic. The electronic configuration of an Atom or molecule is the number of e – (electrons) in the orbit or shells. And electrons of Arsenic is 33, and its . Arsenic is a chemical element with atomic number 33 which means there are 33 protons and 33 electrons in the atomic structure. The chemical symbol for .
Learn the electron configuration of arsenic, a semi-metal with the symbol As and atomic number 33. Find out its properties, uses, toxicity and biological function.
Arsenic (As) has an atomic mass of 33. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.Get the facts about element Arsenic (As) [33] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .
Arsenic ion(As 3-) electron configuration. The ground state electron configuration of arsenic is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3. This electron configuration shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five. The complete electron for a neutral arsenic atom is: 1s22s22p63s23p63d104s24p3. Its shorthand electron configuration is: [Ar]3d104s24p3. Explanation: As is the chemical symbol for the element arsenic. Its atomic number is 33, which is the number of protons in the nuclei of its atoms.A step-by-step description of how to write the electron configuration for Arsenic (As and the As 3- ion).In order to write the As electron configuration we . The information on this page is fact-checked. Arsenic electron configuration | Image: Learnool. The arsenic electron configuration, represented as [ Ar] 4s 2 3d 10 4p 3 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3, showcases the precise arrangement of electrons within the atom.
What is The Electron Configuration of Arsenic. The electronic configuration of an Atom or molecule is the number of e – (electrons) in the orbit or shells. And electrons of Arsenic is 33, and its electron configuration of arsenic is 1s 2 . Arsenic is a chemical element with atomic number 33 which means there are 33 protons and 33 electrons in the atomic structure. The chemical symbol for Arsenic is As. Electron Configuration and Oxidation States of Arsenic. Electron configuration of Arsenic is [Ar] 3d10 4s2 4p3. Possible oxidation states are +3,5/-3. Electron ConfigurationElectron configuration of arsenic. The simplified electron configuration of Arsenic is: [Ar] 3d10 4s2 4p3 and its complete electron configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3. With the Electron configuration, it is possible to know the way in which the electrons are structured in the atoms.Arsenic (As) has an atomic mass of 33. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.

Get the facts about element Arsenic (As) [33] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information.
Stefan V. Jan 6, 2015. The (neutral) atom that has 33 electrons is called Arsenic ( As ), and its electron configuration can be written like this: As:1s22s22p63s23p64s23d104p3. Another way to write the electron configuration for . Arsenic ion(As 3-) electron configuration. The ground state electron configuration of arsenic is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3. This electron configuration shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five.
The complete electron for a neutral arsenic atom is: 1s22s22p63s23p63d104s24p3. Its shorthand electron configuration is: [Ar]3d104s24p3. Explanation: As is the chemical symbol for the element arsenic. Its atomic number is 33, which is the number of protons in the nuclei of its atoms.
A step-by-step description of how to write the electron configuration for Arsenic (As and the As 3- ion).In order to write the As electron configuration we . The information on this page is fact-checked. Arsenic electron configuration | Image: Learnool. The arsenic electron configuration, represented as [ Ar] 4s 2 3d 10 4p 3 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3, showcases the precise arrangement of electrons within the atom.
What is The Electron Configuration of Arsenic. The electronic configuration of an Atom or molecule is the number of e – (electrons) in the orbit or shells. And electrons of Arsenic is 33, and its electron configuration of arsenic is 1s 2 . Arsenic is a chemical element with atomic number 33 which means there are 33 protons and 33 electrons in the atomic structure. The chemical symbol for Arsenic is As. Electron Configuration and Oxidation States of Arsenic. Electron configuration of Arsenic is [Ar] 3d10 4s2 4p3. Possible oxidation states are +3,5/-3. Electron Configuration
Electron configuration of arsenic. The simplified electron configuration of Arsenic is: [Ar] 3d10 4s2 4p3 and its complete electron configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3. With the Electron configuration, it is possible to know the way in which the electrons are structured in the atoms.
Arsenic (As) has an atomic mass of 33. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Play free online games at CrazyGames, the best place to play high-quality browser games. We add new games every day. Have fun!
electron configuration of arsenic|Arsenic electron configuration